Now Enrolling: Solar Clinical Trials
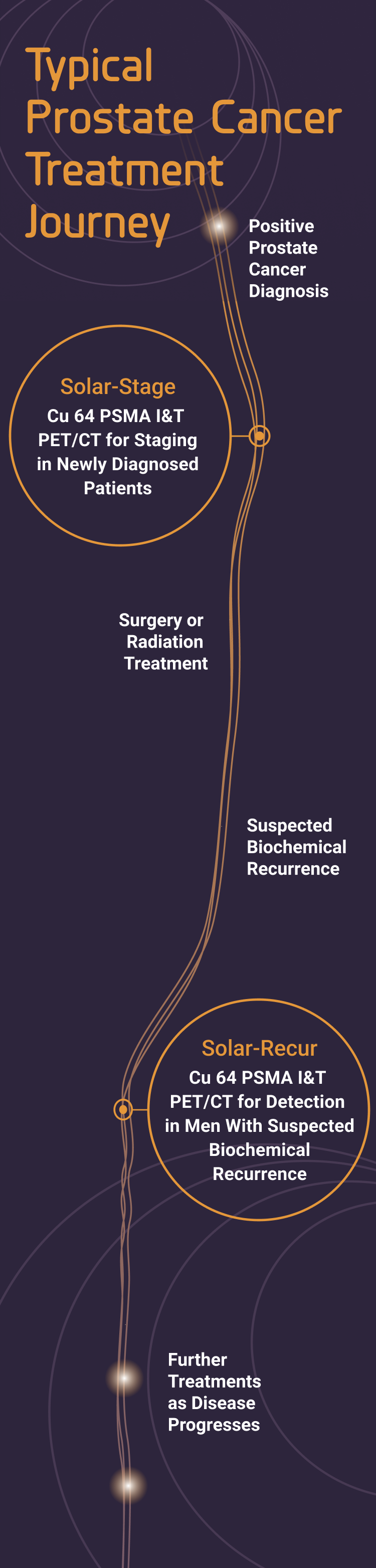
Your patients undergoing staging for newly diagnosed prostate cancer or with suspected biochemical recurrent prostate cancer are invited to participate in Phase 3 clinical trials testing the diagnostic performance of copper Cu 64 PSMA I&T, a new agent for positron emission tomography (PET)/computed tomography (CT) imaging.
Copper Cu 64 PSMA I&T PET/CT imaging could be useful at different stages of a patient’s prostate cancer journey.
Please see each trial page for more information about eligibility.


About Copper Cu 64 PSMA I&T PET/CT
What Is Copper Cu 64 PSMA I&T PET/CT?
Copper Cu 64 PSMA I&T is a radioactive PET/CT imaging agent that specifically targets the prostate-specific membrane antigen (PSMA) protein, which is overexpressed in metastatic prostate cancers.
Copper Cu 64 has a longer half-life (12.7 hours) and shorter positron emission range than many other isotopes, which could enable high-quality image resolution.1,2
Clinical Development Overview
- Copper Cu 64 PSMA I&T PET/CT has been investigated in a Phase 1/2 study of 38 men with recurrent metastatic prostate cancer.
- It was determined in the Phase 2 trial that a single dose of 7-9 mCi copper Cu 64 PSMA I&T PET/CT enabled detection and localization of prostate lesions with sufficient image quality for diagnostic purposes.
- Copper Cu 64 PSMA I&T PET/CT was well tolerated in these studies, with no treatment-related adverse events and no changes in measured safety parameters.
The Phase 3 studies of copper Cu 64 PSMA I&T, a radiopharmaceutical, are being performed for research purposes only. Radiopharmaceuticals contribute to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Cu 64 PSMA I&T is not approved by the United States Food and Drug Administration. The safety and effectiveness of copper Cu 64 PSMA I&T have not been established. The medical benefits and risks of this agent are under investigation.
Resources for Your Patients With mCRPC
Encourage your patients to watch the videos below to learn more about nuclear medicine and how it is being used in prostate cancer.

What Is Nuclear Medicine for Cancer?
Overview of what nuclear medicine is and how it is used to diagnose and treat cancer by targeting tumors with precision.

What Is PSMA for Prostate Cancer?
Introduction to what prostate-specific membrane antigen (PSMA) is, its role in prostate cancer detection and treatment, and how it helps doctors see and target prostate cancer cells.

What Is PSMA Imaging for Prostate Cancer?
Overview of prostate-specific membrane antigen (PSMA)–targeted imaging and how it detects prostate cancer with high accuracy, and its role in diagnosis, staging, and treatment planning.

What Is Nuclear Medicine for Cancer?

What Is PSMA for Prostate Cancer?

What Is PSMA Imaging for Prostate Cancer?
Contact Us
For more information on eligibility and enrollment in the Solar-Stage or Solar-Recur clinical trials, please contact us using the form below.
More information is also
available at ClinicalTrials.gov
(Solar-Stage: NCT06235151,
Solar-Recur: NCT06235099).
References

