
Solar-Stage Clinical Trial
A Phase 3, multicenter, open-label study to test the diagnostic performance of copper Cu 64 PSMA I&T PET/CT in staging of men with newly diagnosed unfavorable intermediate-risk, high-risk, or very-high-risk prostate cancer electing to undergo radical prostatectomy (RP) with pelvic lymph node dissection (PLND).
Solar-Stage
Study Overview
Solar-Stage is a single-arm prospective study. Treating physicians will not be blinded to the imaging results. Local read of the copper Cu 64 PSMA I&T PET/CT can be used to inform patient management. For the co-primary endpoints, PET/CT scans will be read by blinded independent reviewers.
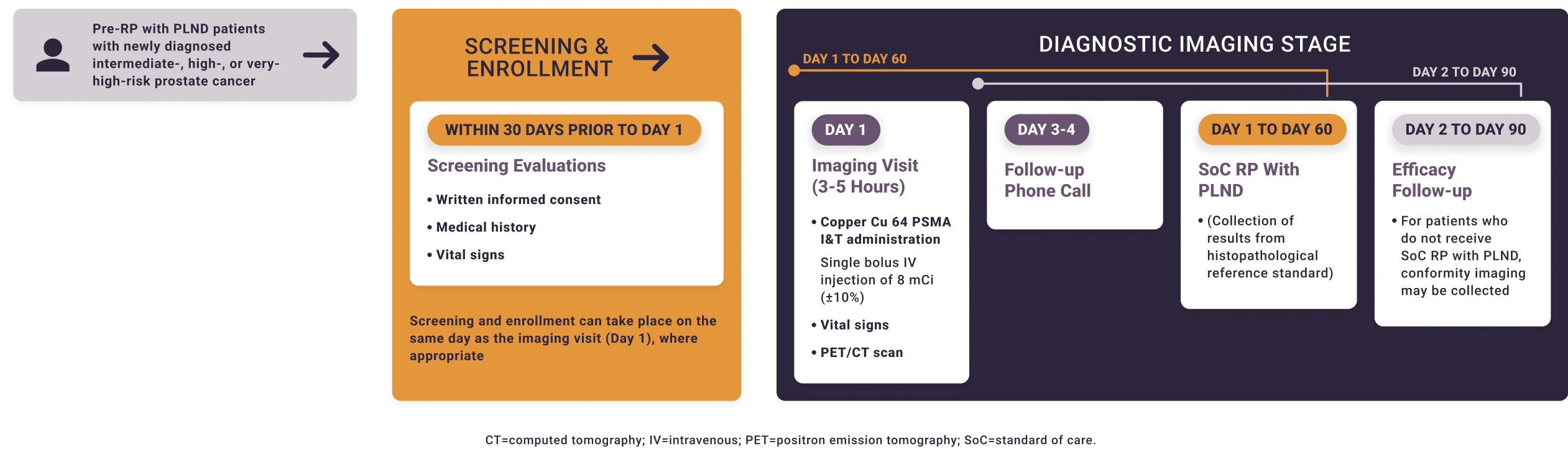
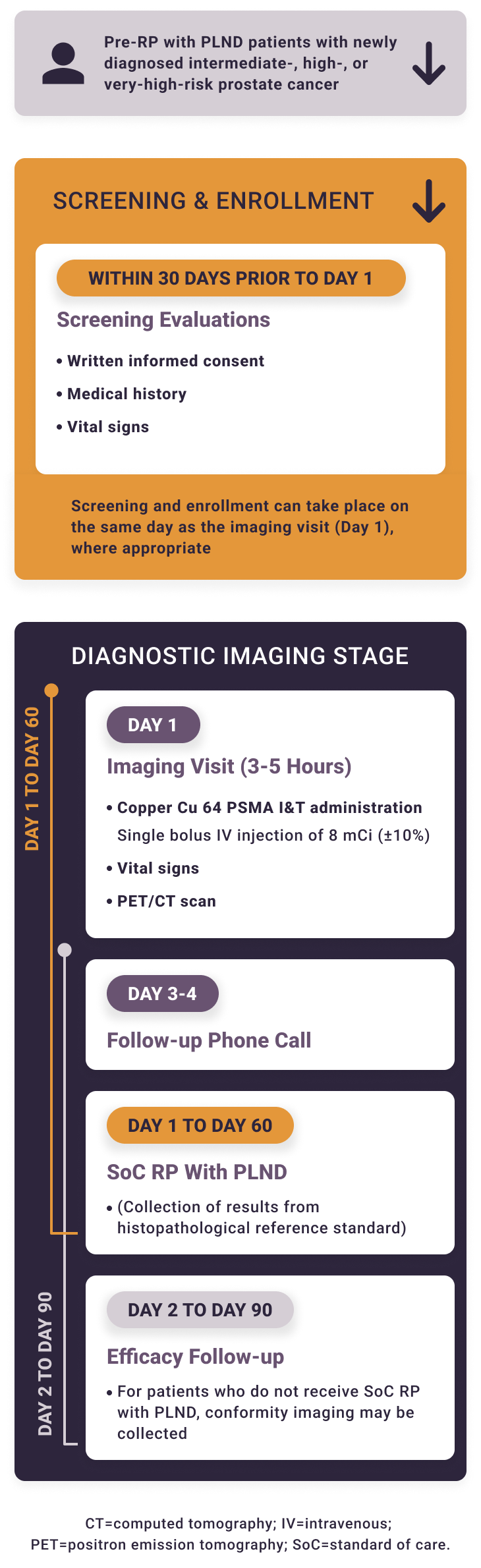
The Phase 3 studies of copper Cu 64 PSMA I&T, a radiopharmaceutical, are being performed for research purposes only. The safety and effectiveness of copper Cu 64 PSMA I&T have not been established by the United States Food and Drug Administration. The medical benefits and risks of this agent are under investigation.
Key Endpoints
- The sensitivity of copper Cu 64 PSMA I&T PET/CT to determine the presence of pelvic lymph nodes relative to histopathology reference standard
- The specificity of copper Cu 64 PSMA I&T PET/CT to determine the absence of pelvic lymph nodes relative to histopathology reference standard




- Inter- and intra-reader agreement of copper Cu 64 PSMA I&T PET/CT scan interpretation by blinded independent readers
- Treatment-emergent adverse events within 72 hours of copper Cu 64 PSMA I&T PET/CT administration
Key Eligibility Criteria
Key Inclusion Criteria
- Male aged ≥18 years
- Histologically proven prostate adenocarcinoma
- Planned RP with extended PLND
- Unfavorable intermediate-risk, high-risk, or very-high-risk disease defined by NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer Version 1.2023 and previous versions NCCN®=National Comprehensive Cancer Network®.
Key Exclusion Criteria
- Androgen deprivation therapy, neoadjuvant chemotherapy, radiation therapy (including local ablation techniques), or any investigational therapy against prostate cancer prior to RP
- PSMA PET within 90 days prior to enrollment
Study Sites
The Solar-Stage study will be conducted at sites across the United States. Click on each state below to see the trial locations and primary investigators.
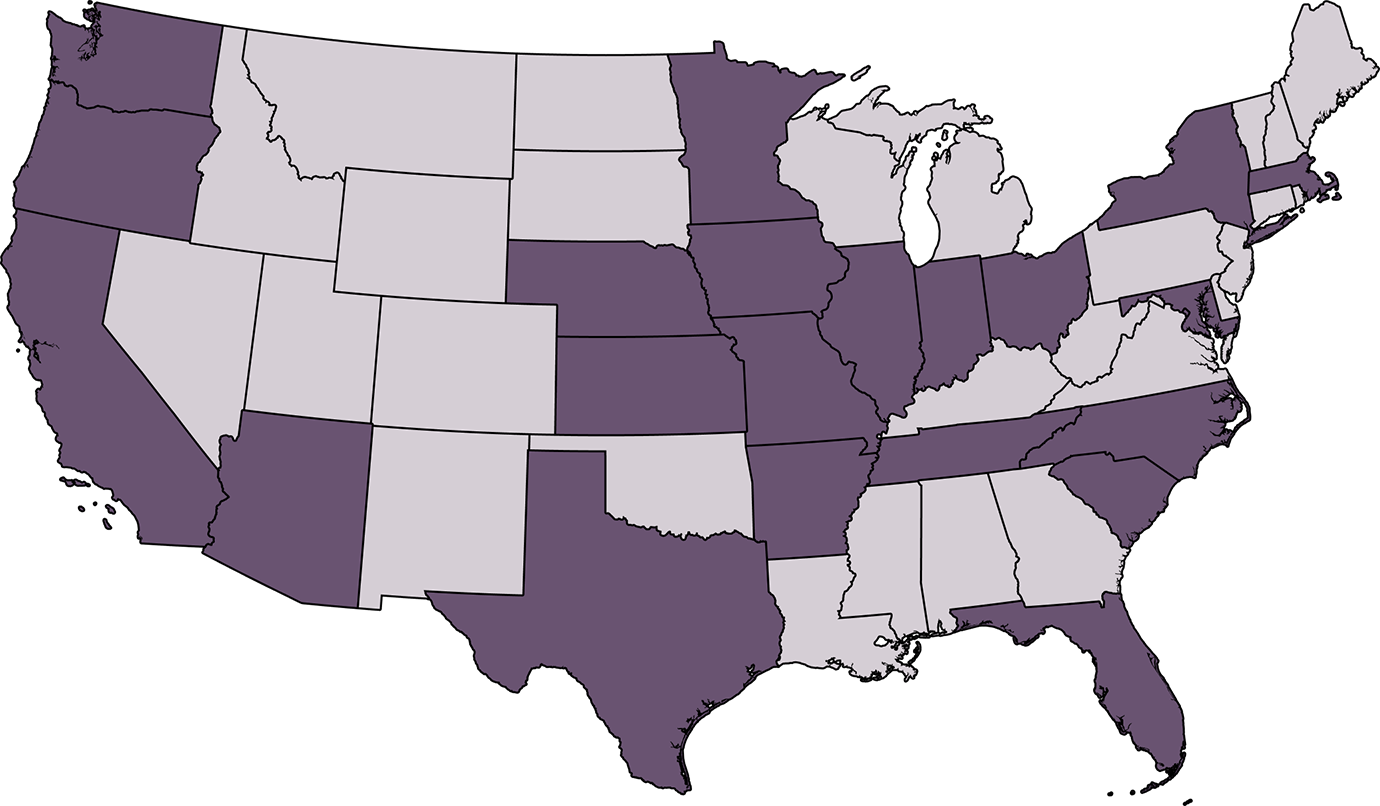
Click on each state below to see the trial locations and primary investigators.
Contact Us
For more information on eligibility and enrollment in the Solar-Stage clinical trial, please contact us using the form below.
More information is also
available at ClinicalTrials.gov
(Solar-Stage: NCT06235151,
Solar-Recur: NCT06235099).